USA, CDC and FDA: 15 cases of thrombosis-thrombocytopenia syndrome (TTS) reported, numbers double in one week
FDA and CDC have recommended lifting the pause on Johnson & Johnson (Janssen) COVID-19 Vaccines in the USA.
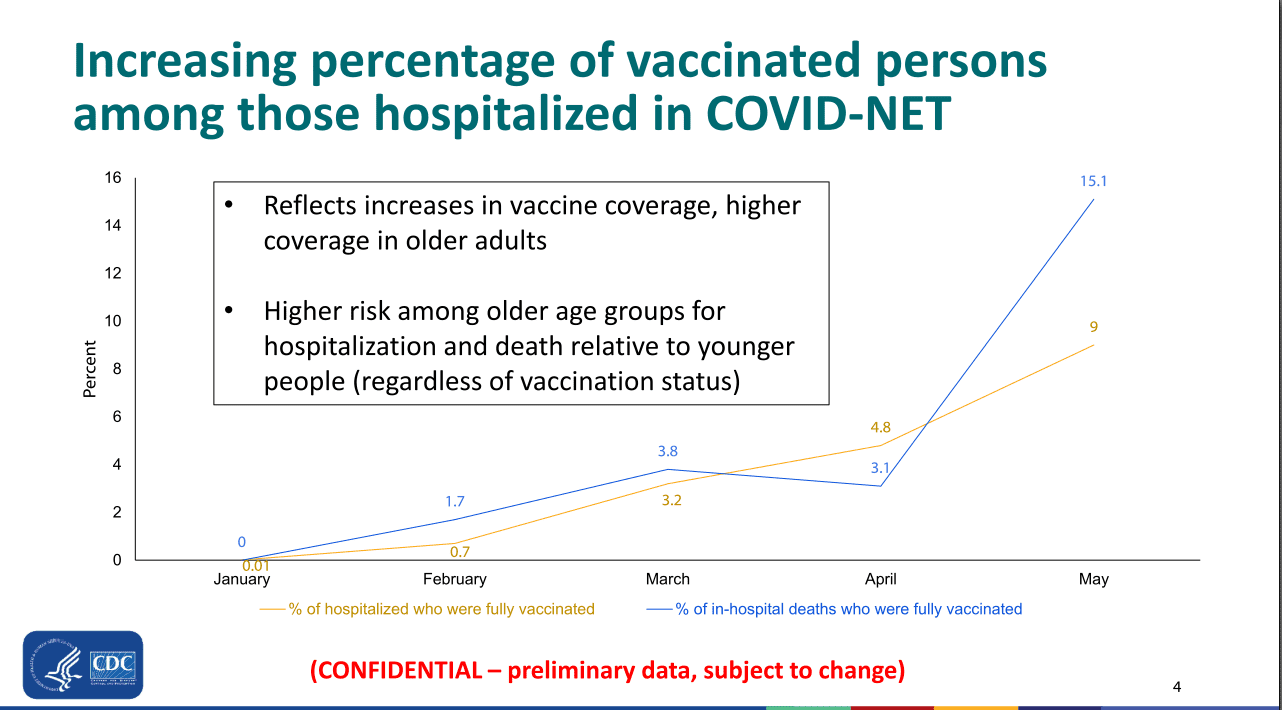
On April 13, the FDA and CDC announced that, out of more than 6.8 million doses administered, six reports of a rare and severe type of blood clot combined with low blood platelet levels occurring in people after receiving the Janssen COVID-19 Vaccine had been reported to VAERS. In these cases, a type of blood clot called cerebral venous sinus thrombosis (CVST) was seen in combination with low levels of blood platelets (thrombocytopenia).
Today, the agencies can confirm that a total of 15 cases of TTS have been reported including the original six reported cases. All of these cases occurred in women between the ages of 18 and 59, with a median age of 37 years. Reports indicated symptom onset between 6 and 15 days after vaccination.