WHO: Rapid Risk Assessment for XBB.1.5 published
The World Health Organisation has issued a rapid risk assessment for the Omicron XBB.1.5 variant (aka Kraken), which we are republishing below.
XBB.1.5 Rapid risk assessment, 11 January 2023
The Omicron XBB.1.5 variant is a sublineage of XBB, which is a recombinant of two BA.2 sublineages.
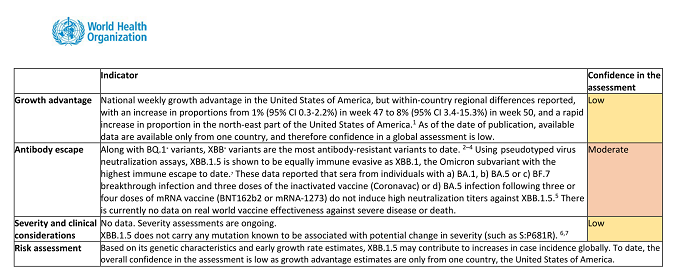
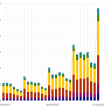
From 22 October 2022 to 11 January 2023, 5,288 sequences of the Omicron XBB.1.5 variant have been reported from 38 countries. Most of these sequences are from the United States of America (82.2%), the United Kingdom (8.1%), and Denmark (2.2%).
WHO’s Technical Advisory Group on Virus Evolution (TAG-VE) met on 5 January 2023 to discuss the latest evidence on XBB.1.5 and assess the public health risk associated with this variant. Based on its genetic characteristics and early growth rate estimates, XBB.1.5 may contribute to increases in case incidence. To date, the overall confidence in the assessment is low as growth advantage estimates are only from one country, the United States of America.
WHO and the TAG-VE recommend Member States to prioritize the following studies to better address uncertainties relating to the growth advantage, antibody escape, and severity of XBB.1.5. The suggested timelines are indicative and will vary from one country to another based on national capacities:
- Analysis of growth advantage from additional countries where XBB.1.5 has been detected (1-3 weeks).
- Neutralization assays using human sera representative of the affected community(ies) and XBB.1.5 live virus isolates (2-6 weeks).
- Comparative assessment to detect changes in rolling or ad hoc indicators of severity (see table below, 4-12 weeks)
The rapid risk assessment below is based on currently available evidence and will be revised regularly as more evidence and data from additional countries become available.
Growth advantage
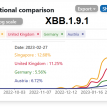
National weekly growth advantage in the United States of America, but within-country regional differences reported, with an increase in proportions from 1% (95% CI 0.3-2.2%) in week 47 to 8% (95% CI 3.4-15.3%) in week 50, and a rapid increase in proportion in the north-east part of the United States of America. 1 As of the date of publication, available data are available only from one country, and therefore confidence in a global assessment is low.
Antibody escape
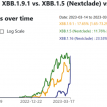
Along with BQ.1 * variants, XBB * variants are the most antibody-resistant variants to date. 2–4 Using pseudotyped virus neutralization assays, XBB.1.5 is shown to be equally immune evasive as XBB.1, the Omicron subvariant with the highest immune escape to date. 7 These data reported that sera from individuals with a) BA.1, b) BA.5 or c) BF.7 breakthrough infection and three doses of the inactivated vaccine (Coronavac) or d) BA.5 infection following three or four doses of mRNA vaccine (BNT162b2 or mRNA-1273) do not induce high neutralization titers against XBB.1.5. 5 There is currently no data on real world vaccine effectiveness against severe disease or death.
Severity and clinical considerations
No data. Severity assessments are ongoing. XBB.1.5 does not carry any mutation known to be associated with potential change in severity (such as S:P681R).
Risk assessment
Based on its genetic characteristics and early growth rate estimates, XBB.1.5 may contribute to increases in case incidence globally. To date, the overall confidence in the assessment is low as growth advantage estimates are only from one country, the United States of America.
11jan2023_XBB15_rapid_risk_assessment-1