Category: Slovakia
West European summer Covid wave brewing *6 updates, 1 prediction*
A new western European summer Covid wave appears to be developing, with increases in cases in countries that also saw large waves in the summer of 2021. More...
Spillover of SARS-CoV-2 across Europe from the war in Ukraine
This is a new post that will be tracking the possible spillover of SARS-CoV-2 from the war zone in Ukraine and into neighbouring countries. More...
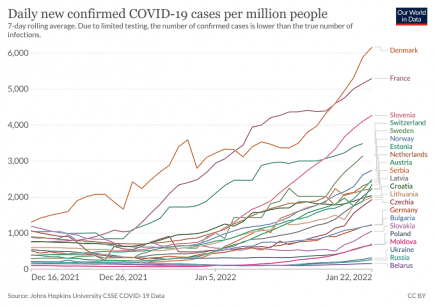
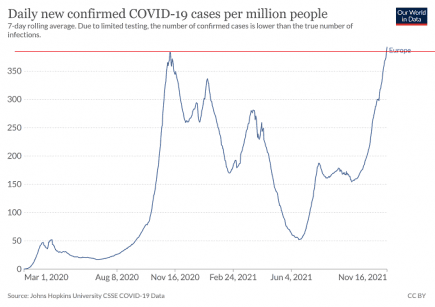
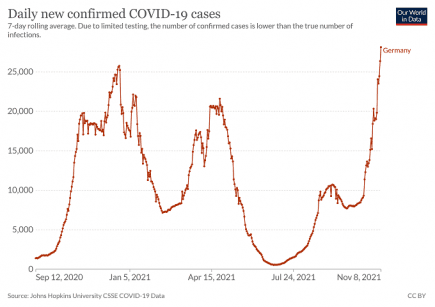
Europe: Another huge Covid wave is building right across the continent
Almost every country in Europe is now seeing a big increase in Covid cases. More...
Slovakia: Two week Covid lockdown introduced
Slovakia will be put in lockdown for two weeks from Thursday, November 25th 2021. More...
Slovakian President: “We are losing the fight against the pandemic, we need a lockdown”
“We are losing the fight against the pandemic, we need a lockdown”, said President Zuzana Čaputová on Tuesday after visiting the hospital in Ružinov in Bratislava. More...
Slovakia Health Minister calls for three week full lockdown
The Slovakian Minister of Health, Vladimír Lengvarský, is calling for a three-week Covid lockdown for everyone. More...
Europe: all-time Covid infection record broken by growing winter superwave
Europe has broken through its all-time high for Covid cases, and is seeing sharp increases in infections right across the continent. More...
Europe: explosive Covid growth rates, steeper than the winter wave of 2020 in many countries
Europe is seeing some stunning increases in the daily Covid case rate across much of the continent. More...
Prediction: European Covid winter wave of 2021 will be the worst wave of the pandemic so far
This isn’t the most difficult prediction we have ever had to make, but there is no doubt that, despite huge vaccination campaigns across the continent, the winter wave now breaking over Europe will be their worst wave of the pandemic so far. More...
WHO: 500,000 deaths from Covid in Europe this winter
“The current pace of transmission across the 53 countries of the European Region is of grave concern,” said regional WHO head Hans Kluge. More...